CBD is nonpsychoactive, nonaddictive, does not produce a "high" and has few to no dangerous side effects
In states where CBD is becoming widely used, there are also few reports of negative social or medical consequences; in fact, CBD has been shown to provide valuable benefits for those struggling with opioid addiction
While the sale of CBD products has exploded into a $390 million per year industry and is projected to hit $1.3 billion by 2022, there’s still a lot of confusion around the federal legality of CBD commerce in the U.S.
Hemp was legalized under the 2018 Farm Bill, and FDA has reclassified CBD as a Schedule 5 drug with low risk of addiction. However, since FDA has approved a CBD-only drug, CBD is no longer recognized as a dietary supplement and cannot legally be sold as such
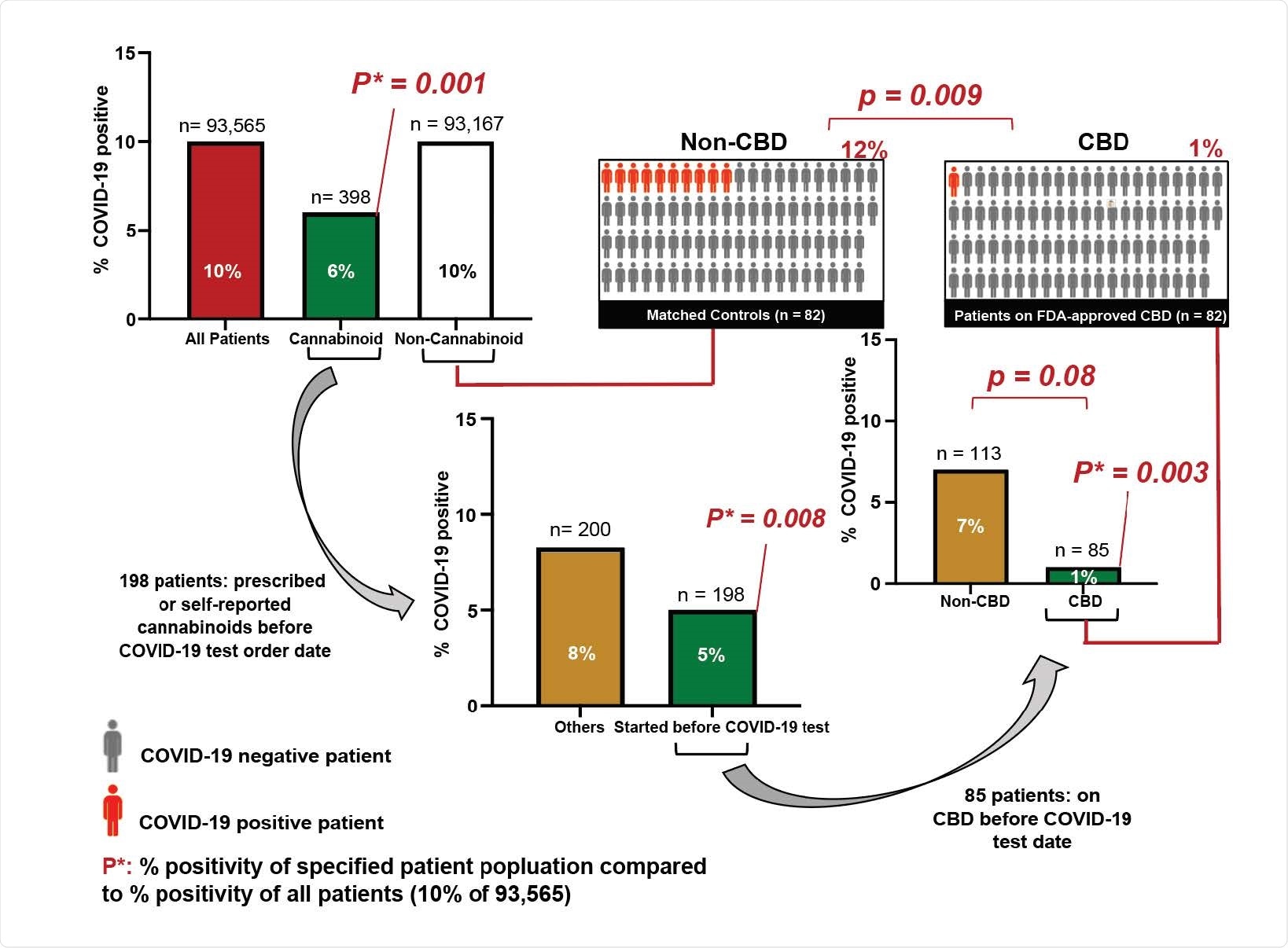
There’s need for quality control; in one study, 26.19% of 84 CBD products tested contained less CBD than advertised, and 42.85% of them contained more. Only 30.95% were accurately labeled
Production of cannabis is booming as the medical benefits of CBD (cannabidiol) are increasingly recognized. According to Project CBD, at least 50 conditions are believed to be improved by CBD, including pain, seizures, muscle spasms, nausea associated with chemotherapy, digestive disorders, degenerative neurological disorders such as multiple sclerosis and Parkinson's disease, mood disorders, anxiety, PTSD and high blood pressure.
CBD is non psychoactive, nonaddictive, does not produce a "high" and has few to no dangerous side effects. In states where CBD is becoming widely used, there are also few reports of negative social or medical consequences, in fact, CBD has been shown to provide valuable benefits for those struggling with opioid addiction.
At the end of 2018, the U.S. Food and Drug Administration modified the Schedule 1 classification for some CBD products, downgrading CBD products from cannabis that contain no more than 0.1%tetrahydrocannabinols (THC, the psychoactive component of cannabis) to Schedule 5, which lists drugs considered to have a lower potential for abuse than other controlled drugs.
The popularity of CBD has exploded in recent years. As noted in a May 14, 2019, New York Times article:
“ … [C]annabidiol is everywhere. We are bombarded by a dizzying variety of CBD-infused products: beers, gummies, chocolates and marshmallows; lotions to rub on aching joints; oils to swallow; vaginal suppositories … CVS and Walgreens each recently announced plans to sell CBD products in certain states.”
Discovery of the Human Endocannabinoid System
The New York Times, which covers a lot of ground in its article, goes on to recount some of the history of how scientists discovered what cannabis can do for the human body. In the early 1960s, a chemist named Raphael Mechoulam became the first to map the chemical structures of CBD and THC.
In the 1980s, a St. Louis University Medical School scientist named Allyn Howlett identified the cannabinoid receptor type 1 (CB1) in the human brain. We now know there are two types of cannabinoid receptors in the human body, CB1 and CB2, and while CB1 is typically thought of as being primarily in the brain and CB2 primarily in the immune system, both types of receptors are in fact found throughout your body.
We also know the body produces endogenous cannabinoids that influence these receptors, and that this endocannabinoid system (ECS) plays an important role in human health, as it regulates homeostasis by orchestrating communication between your bodily systems, such as your respiratory, digestive, immune and cardiovascular systems.
Mechoulam published his discovery of the first endogenous cannabinoid, anandamide, which attaches to the same sites as THC, in 1992. As you’d expect, the discovery of the ECS led to drug developments targeting CB receptors.
Results, however, were less than fruitful — turns out there are few truly safe alternatives to cannabis (or hemp, which also contains CBD, but very little if any THC). As reported by The New York Times,Sanofi’s weight loss drug Rimonabant, which blocked CB1 receptors, was pulled off the market after just two years, after reports of depression and suicide attempts related to the drug emerged.
“The episode seems to exemplify endocannabinoids’ importance to our sense of well-being and the difficulty of manipulating them therapeutically. Attempts to increase native cannabinoids with synthetic drugs have fared no better.
In 2016, French scientists halted a study of a drug designed to boost endocannabinoids. For reasons that remain unclear, six patients who took the medicine, meant to treat pain, were hospitalized. One died,” The New York Times writes.
How CBD Affects Your Body
While there’s still a lot we don’t know about the exact workings of CBD, research suggests it interacts with many different systems in the body. For example, it increases the number of endogenous cannabinoids, binds to serotonin receptors and stimulates GABA receptors, all of which play roles in mood.
“With more than 65 cellular targets, CBD may provide a kind of full-body massage at the molecular level,” The New York Times states, adding, “this biochemical promiscuity is one reason CBD seems so medically promising,” especially when it comes to neurology, as CBD’s ability to re-establish homeostasis appears particularly beneficial for the brain.
While the featured New York Times article centers around the story of a mother seeking to find a medical solution for her son who has severe epilepsy, it also touches on many other medical uses, including current research investigating the use of CBD as a prophylactic to prevent schizophrenia.
Early warning signs of schizophrenia include episodes of delusions, during which the patient is still aware that the experiences aren’t real. A study published in 2018 found giving these patients a single 600-milligram dose of CBD helps to normalize some of the abnormal function in the parahippocampal, striatal and midbrain areas that occur during a schizophrenic episode.
Cannabis Products May Be an Answer to the Opioid Epidemic
While THC is psychoactive (creates a “high”), CBD has been shown to counteract the effects of THC. Many cannabis varieties used for recreational purposes, however, have been bred for exceptionally high THC content, which is why recreational marijuana use has become associated with some of the more negative effects of THC.
A study on black-market marijuana published in 2016 revealed the THC potency of illicit marijuana has “consistently increased over time since 1995 from ~4% in 1995 to ~12% in 2014,” while “cannabidiol content has decreased on average from ~.28% in 2001 to <.15% in 2014.”
This means the overall change in the THC to CBD ratio has shifted from 14-to-1 to about 80-to-1 over the past couple of decades. Older varieties of cannabis had a ratio closer to 1-to-1.
What’s more, while THC has been shown to trigger heroin-seeking behavior, CBD has been found to do the complete opposite, which is why CBD is now being investigated as an aid to end addiction to opioids.
The research, The New York Times states, “indicates that CBD might help recovering opioid addicts avoid relapse, perhaps the greatest challenge they face … And because it’s not habit-forming … CBD might be a badly needed new weapon with which to fight an epidemic that claims more than 130 lives daily in the United States.”
A recent study published in the American Journal of Psychiatry confirms the benefits of CBD for this use yet again, finding:
“Acute CBD administration, in contrast to placebo, significantly reduced both craving and anxiety induced by the presentation of salient drug cues … CBD also showed significant protracted effects on these measures 7 days after the final short-term (3-day) CBD exposure.
In addition, CBD reduced the drug cue-induced physiological measures of heart rate and salivary cortisol levels. There were no significant effects on cognition, and there were no serious adverse effects.”
THC is not all bad though. Certain kinds of pain respond better to THC than CBD, and research has shown cancer patients who use cannabis need fewer opioids to manage their pain. THC also appears to be less toxic to older brains, and has been shown to actually rejuvenate the aging brain and reduce plaque buildup associated with Alzheimer’s.
States that have legalized medical cannabis also report fewer opioid-related deaths. Research published in 2014 showed that, on average, opioid overdose mortality in these states is 24.8% lower than in states that do not have medical cannabis laws, and that mortality rates from opioids continue to decline over time.
Current Cannabis Drugs
At present, there are two cannabis-related drugs on the market. The first, Marinol, contains synthetic THC and is prescribed for the suppression of nausea and appetite stimulation.
The second, Epidiolex, approved by the FDA in June 2018, is the first CBD-only drug derived directly from the cannabis plant. The featured New York Times article details the behind-the-scenes story of how this drug was developed and brought to market.
The FDA’s downgrading of CBD with minimal THC content to a Schedule 5 drug was in direct response to its approval of Epidiolex, which is approved for the treatment of Lennox-Gastaut syndrome and Dravet syndrome, two rare forms of epilepsy.
As explained by The New York Times, the impetus behind the development of this particular drug was two mothers who were experimenting with CBD as treatment for their children’s epilepsy and then got GW Pharmaceuticals involved.
"'In the modern era, it’s certainly the most striking example of a drug that has gone from patient use to drug development,' Ken Mackie, a neuroscientist at Indiana University, told me.
And it’s unlikely to be the last such example. Because so many people already use cannabis and think it helps, patients might be, in effect, pioneering new uses through self-experimentation,"Moises Velasquez-Manoff writes in The New York Times, adding:
"[M]any who have direct experience with CBD, including a few scientists, do not think it should be available only by prescription. They point out that long before the 1970 Controlled Substances Act, which made marijuana illegal, people used the plant medicinally. Cannabis should not only take its place as an F.D.A.-approved drug, they contend. It should also reclaim its role as a folk remedy."
Quality Control Is an Issue
While it seems clear CBD can be beneficial for a range of health issues, quality and dosing are important issues that cannot be overlooked. It’s also important to understand that it may not work for everyone.
Dr. Elizabeth Thiele, an epileptologist at Harvard, told The New York Times some children experience mood changes on some nonprescription CBD products, and that it can interfere with other medications by changing the rate at which your body breaks them down.
The need for more stringent quality control has already been demonstrated in studies showing 26.19% of 84 CBD products tested contained less CBD than advertised, and 42.85% of them contained more. Only 30.95% were accurately labeled.
An earlier study found many medical cannabis products sold in dispensaries in California and Washington contained very little CBD, and only 17% were accurately labeled with regard to THC and CBD content; 23% were under labeled and 60% were over labeled with respect to THC. According to the authors:
“The median THC:CBD ratio of products with detectable CBD was 36:1, 7 had ratios of less than 10:1, and only 1 had a 1:1 ratio. Edible cannabis products from 3 major metropolitan areas, though unregulated, failed to meet basic label accuracy standards for pharmaceuticals.
Greater than 50% of products evaluated had significantly less cannabinoid content than labeled, with some products containing negligible amounts of THC. Such products may not produce the desired medical benefit.
Other products contained significantly more THC than labeled, placing patients at risk of experiencing adverse effects. Because medical cannabis is recommended for specific health conditions, regulation and quality assurance are needed.”
As of January 16, 2019, new cannabis regulations took effect in California, which include more stringent quality controls. As a result, patients are more likely than ever to get what they pay for from licensed dispensaries in the state. Quality controls include testing for microbes, pesticides and heavy metals.
Heavy metal testing is particularly important for hemp-based CBD products, as the plant is known to extract heavy metals from the soil. In fact, it’s frequently used for bioremediation purposes, which is great if the hemp is used for rope, fuel and other non medical uses.
When made into medicine, however, this soil-cleansing feature could pose significant problems, as it must be grown in clean soil. As a general rule, I recommend seeking out certified organic CBD products to ensure the least amount of contamination with pesticides and other harmful agricultural contaminants.
Legality of CBD Commerce Remains Confusing
While the sale of CBD products has exploded into a $390 million per year industry and is projected to hit $1.3 billion by 2022, there’s still a lot of confusion around the federal legality of CBD commerce in the U.S.
As noted by STAT News, “You wouldn’t know it from their widespread availability on the internet, in health food shops, and increasingly in major retailers, but CBD dietary supplements are technically illegal.” The reason for this is because the FDA still has not clarified whether CBD should be considered a drug or a supplement.
Hemp can now be grown and sold legally per the 2018 Farm Bill, but according to the FDA, CBD from hemp “is still technically illegal as a dietary ingredient,” New Hope Network points out. By approving a CBD-only drug (Epidiolex), CBD cannot — per FDA rules — be sold as a supplement, even if it’s derived from legal hemp.
“Lawyers, however, maintain the law is less clear for CBD products marketed as hemp extract, because hemp has been in the food supply long before drug makers started doing CBD clinical trials,” STAT News says. For now, FDA has not aggressively gone after CBD products — limiting their action to gong after those making unsubstantiated disease claims — but they could, and this makes many doctors nervous.
“J. Michael Bostwick, a psychiatrist at the Mayo Clinic, in Rochester, Minn., who has written about cannabis, calls the hodgepodge of conflicting rules regarding cannabis ‘idiotic,’”Velasquez-Manoff writes.
“He told me that even physicians willing to oversee patient cannabis use, who live in states where it’s legal, can be reluctant to do so because it remains illegal under federal law.
A doctor’s license to practice medicine comes from the state, but because the license that allows doctors to prescribe medicine is federal, involvement with cannabis could lead to revocation of that license. ‘There’s a lack of clarity about what playing field we’re on,’ Bostwick says.”
What Will the Future of CBD Regulations Hold?
While making CBD products an FDA approved drug is one solution, it’s by far not the only one, Velasquez-Manoff notes in his New York Times article. Quality can be ensured in other ways.
In Germany, for example, medical marijuana is overseen by a federal agency responsible for making sure all products are pharmaceutical grade. The Canadian company Tilray also produces medical-grade cannabis products and flowers and ships to any location where they’re federally legal (which currently excludes the U.S.). According to STAT News:
“[T]he FDA is considering whether it can write a new regulation that would carve CBD out of the existing law. The agency has set up a CBD working group headed by the agency’s principal deputy commissioner, Dr. Amy Abernethy, and the agency’s principal associate commissioner for policy, Lowell Schiller.
The group will be tasked with answering that question. And that same question is sure to loom large at the agency’s May 31 public meeting on CBD — the first of its kind.
‘A key of goal of our upcoming hearing and associated public docket is to identify and collate all available data to help us answer these questions in order to make sure that the American public is protected – including to the extent CBD is being introduced into our food supply or other common consumer products,’ FDA’s Felberbaum said.
It’s entirely new territory for the government. ‘We’ve never done this before,’ Gottlieb said in March. And Gottlieb has warned that the FDA’s process could take longer than three years — a lengthy timeline for an industry that has already seen such rapid growth.
In the meantime, he’s pushed Congress to step in and solve the problem more quickly. ‘The most efficient way to get to a pathway would be through legislation,’ Gottleib said in March. ‘Probably that would just be legislation that would specifically address CBD.’”
The need for clarity was recently highlighted when a 69-year-old woman was arrested and spent 12 hours in jail after Disney World security found CBD oil in her purse. The woman said she used it to alleviate arthritis pain. CBD is legal in her home state of North Carolina, widely available in stores across Florida, and she also had a doctor’s note for the CBD oil.
While the drug charges against her were dropped, the question of whether she should have been arrested in the first place loom large. The woman’s attorney has stated he will pursue legal action against Orange County Sherriff’s office and Disney World for “illegal detention, false arrest and violation of her civil rights.”
Who knows, if that case moves forward, it may prompt some clarity or set some kind of legal precedent. Until then, it’s important to realize that even though CBD products are readily available, from a federal standpoint, they’re illegal, so it would be wise not to travel with them.
Feeling lethargic or unproductive lately? You may want to look at your sleep habits. Getting enough high-quality sleep is a major factor in improving your mood and overall health. Yet a lot of people fall short on it, increasing their risk for various health problems.
If this is an issue you’re struggling with, Sleep 101 is a guide you just might need. This page will open your eyes to the health benefits of sleep and give you tips on how to get enough. Discover how you can cultivate an environment that will help you to drift off to dreamland easily, so you will always wake up feeling refreshed and invigorated.